Abstract
Background: Blinatumomab induces a high proportion of complete molecular responses in relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and ALL with persistent or recurrent measurable residual disease (MRD) after initial therapy. However, responses are generally short if they are not followed by allogeneic stem cell transplantation (HSCT). We infer that blinatumomab needs to be incorporated sequentially into the early phase of treatment to enhance rates of MRD negativity, lower the need of HSCT and improve survival.
Methods: Induction therapy in the Blina-CELL Phase 2 trial (NCT04554485) consisted of run-in phase (dexamethasone 10 mg/m2 days 1-7, cyclophosphamide 200 mg/m2 days 3-5, vincristine 2 mg day 6, and daunorubicin 45 mg/m2 days 6-7), first induction phase (blinatumomab 9 µg/day days 12-19 and 28 µg/day days 20-40), and second induction phase (dexamethasone 10 mg/m2 days 50-54, vindesine 3 mg/m2 day 50, methotrexate 1.5 g/m2 day 50, etoposide 250 mg/m2 days 53-54, and cytarabine 2x 2 g/m2 day 54). Consolidation chemotherapy was identical to the GMALL 07/2003 protocol. Intensive intrathecal prophylaxis was given in both induction and consolidation phases. The primary endpoint was MRD evaluated by quantification of IG/TR rearrangements at week 11 (at the end of induction II). The minimum sensitivity and quantitative range of all assays was 10e-4.
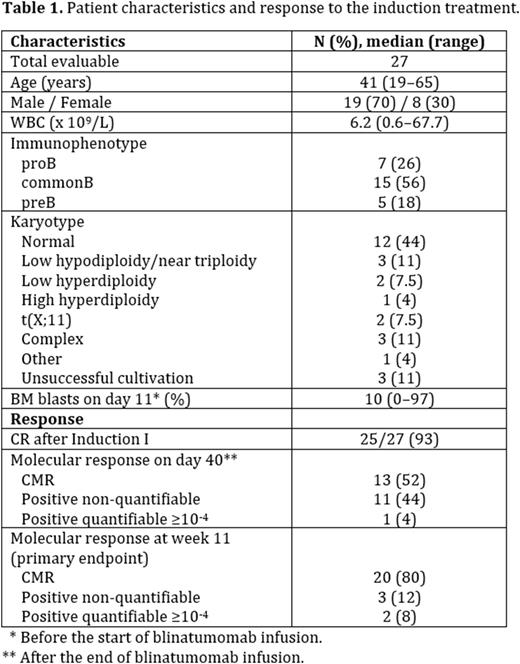
Results: Twenty-nine patients started this treatment between May 2019 and March 2022. One patient terminated the study after 3 days of blinatumomab infusion due to elevated liver function tests, one was considered a screening failure due to the missing MRD target. Twenty-seven patients were evaluable. Median age was 41 years (range, 19-65). Median white blood cell count (WBC) at diagnosis was 6.2 x 109/L (range, 0.6-67.7). Twenty-five patients (93%) achieved complete remission (CR) at the end of induction I, 2 (7%) were refractory (both had BCR::ABL1-like phenotype). No patient died during the induction phase.
Of the 25 patients in CR, 13 (52%) had complete molecular response (CMR) at day 40 (upon termination of blinatumomab infusion), 11 (44%) had positive non-quantifiable MRD and one (4%) patient had quantifiable MRD >10e-4. Primary endpoint assessment at week 11 showed 20 (80%) patients in CMR, 3 (12%) with positive non-quantifiable MRD and 2 (8%) with MRD ≥10e-4.
Four (16%) patients underwent HSCT due to MRD failure or reappearance, all were pre-treated with a further 1-2 cycles of blinatumomab. Three of them (75%) entered HSCT in CMR. One patient was transplanted in CMR for high-risk features (proB, KMT2A rearrangement).
The follow-up period is still ongoing. With 18 months of median follow, the overall and relapse-free survival in the study is 18.2 and 15.9 months, respectively.
The most frequent grade 3-4 adverse events of blinatumomab therapy were: febrile neutropenia (15%), elevated ALT/AST (11%), soft tissue infection (7%) and low fibrinogen (7%). Only one case of grade 3 cytokine release syndrome was documented. No neurotoxicity events were observed during the induction phase of treatment.
Conclusion: Remission induction strategy combining one cycle of blinatumomab with one cycle of high-dose chemotherapy provides a high molecular response rate (CMR or non-quantifiable MRD) of 92%. This approach translates into a low transplant rate due to a high MRD response and has a favourable safety profile. Longer follow-up is needed to evaluate survival benefit.
Disclosures
Salek:Incyte: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Folber:Janssen: Consultancy; Kite Gilead: Consultancy, Honoraria, Other: Travel Grant; Neovii: Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Grant. Hrabovsky:Amgen: Honoraria. Koristek:AbbVie: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Horacek:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Soukup:Novartis: Consultancy, Honoraria. Benkova:Novartis: Consultancy, Honoraria. Cetkovsky:Novartis: Consultancy. Doubek:AbbVie: Honoraria; Janssen: Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; AOP Orphan: Consultancy, Research Funding.
OffLabel Disclosure:
Blinatumomab in the induction therapy for acute lymphoblastic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal